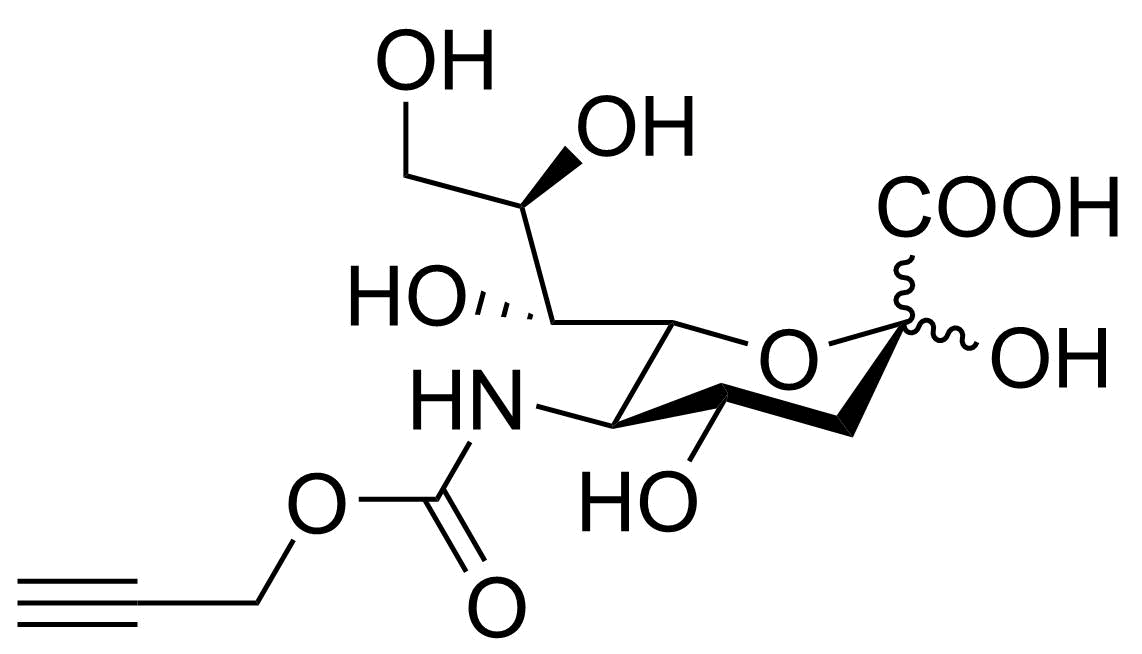
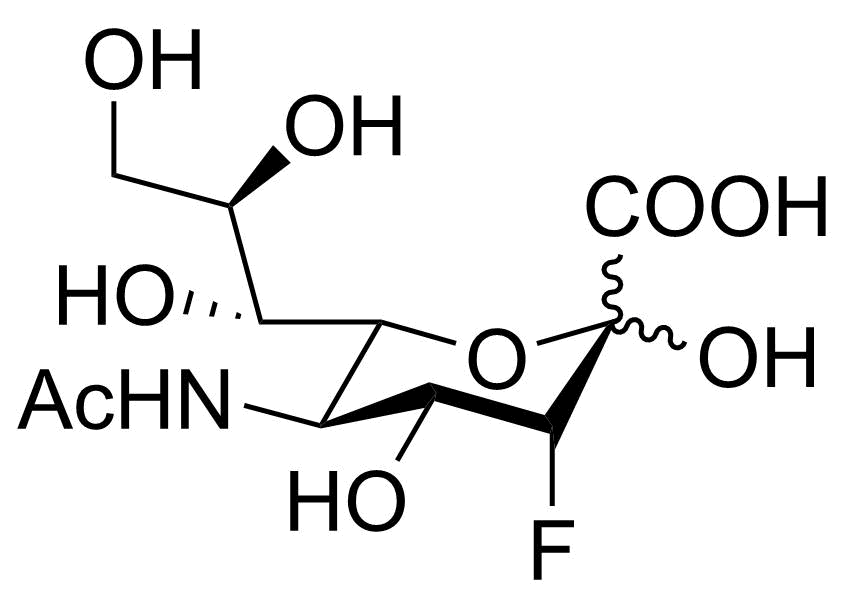
Neu5Ac3F
Metabolic inhibitor of sialylation for active uptake
| Product ID: | SV2524 |
|---|---|
| Synonyms: | 3FaxNeu5Ac; FNANA |
| Tags: | Inhibitor, Neuraminic acid, NTHi, Sialic acid |
| Product | Price | Estimated Shipping Time | Purchase |
|---|---|---|---|
| Neu5Ac3F - 1 mg | €250.00 | 1-3 days | |
| Neu5Ac3F - 5 mg | €450.00 | 1-3 days | |
| Neu5Ac3F - 10 mg | €775.00 | 1-3 days | |
| Neu5Ac3F - 25 mg | €1,650.00 | 1-3 days |
Product information
-
Function
Neu5Ac3F inhibits the sialic acid biosynthesis. It is a global sialylation inhibitor which inhibits the formation of all sialic acid types. It blocks the action of sialyltransferase enzymes. Neu5Ac3F needs to be taken up via a sialic acid transporter. Neu5Ac3F is not taken-up at µM concentrations in mammalian cells since these lack an active transport mechanism. If inhibition of sialylation via passive diffusion is desired, we recommend the use of P-Neu5Ac3F which is taken up via passive diffusion
-
Mode of action
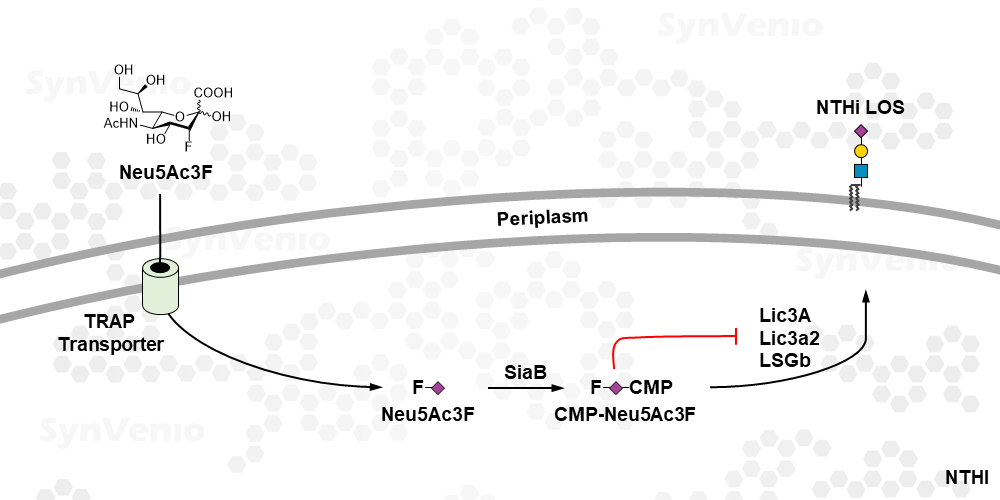
Neu5Ac3F is a metabolic pro-drug. It enters cells via active uptake by the transport system in NTHi (non-typeable Haemophilus influenzae) and is converted to the active inhibitor, CMP-Neu5Ac3F inside NTHi cells. This compound blocks the action of sialyltransferase enzymes and prevents the sialylation of the lipooligosaccharide in NHTi.1 Neu5Ac3F is not taken-up at µM concentrations in mammalian cells since these lack an active transport mechanism. If inhibition of sialylation via passive diffusion is desired, we recommend the use of P-Neu5Ac3F which is taken up via passive diffusion.
-
Applications
Neu5Ac3F is a very good alternative for experiments with Sialidase. Often these enzymes are of bacterial origin and hence easily contaminated, Neu5Ac3F is chemically pure. Furthermore, the effect of a sialidase treatment is short-lived as the cells quickly reproduce sialylated glycans, Neu5Ac3F induces a prolonged suppression of sialylation. Neu5Ac3F has been used to investigate the role of sialylation in NTHi infections in vitro.1
-
Handling
Neu5Ac3F is soluble in water and PBS and is added to cell culture from a stock solution. Desialylation can be assayed with SNA and MALII lectins binding to α-2,6 and α-2,3 linked sialic acids, respectively. Alternatively, a click chemistry based competitive assay can be used to measure the extent of cellular sialylation in NTHi. For this purpose Neu5NAz or Neu5NPoc and a suitable biotin probe can be used.
-
Chemical Information
CAS No.: 921-40-4
SMILES: OC1(C(O)=O)[C@H](F)[C@H](O)[C@@H](NC(C)=O)[C@H]([C@H](O)[C@H](O)CO)O1
Chemical formula: C11H18FNO9
Molecular weight: 327.26
Purity: > 95%
Identity: 1H NMR
Shipping temperature: 20°C
Storage temperature: -20°C
Recommended Products
| Name |
|---|
| P-GalNAz |
| Neu5NPoc |
| XylNap |
| P-GlcNAc6Az |
| XylPNP |
Calculator
Dissolve the required mass in your desired stock volume.
Dilute the required volume of your stock solution to the desired final volume.