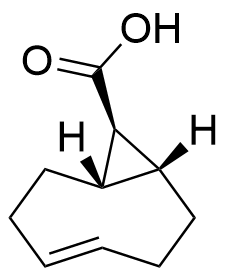
(exo)-sTCO-COOH
Strained trans-cyclooctene with a free carboxylic acid
| Product ID: | SV7054 |
|---|---|
| Synonyms: | exo-sTCO, exo-sTCO-COOH, exo-(E)-BCN, (1R,8S,9r,E)-bicyclo[6.1.0]non-4-ene-9-carboxylic acid |
| Tags: | Alkyne, Bioorthogonal chemistry, click chemistry, copper free click chemistry, TCO, tetrazine, trans-cyclooctene |
| Product | Price | Estimated Shipping Time | Purchase |
|---|---|---|---|
| (exo)-sTCO-COOH - 1 mg | €130.00 | 2-3 weeks | |
| (exo)-sTCO-COOH - 5 mg | €165.00 | 2-3 weeks | |
| (exo)-sTCO-COOH - 10 mg | €200.00 | 2-3 weeks | |
| (exo)-sTCO-COOH - 25 mg | €350.00 | 2-3 weeks | |
| (exo)-sTCO-COOH - 50 mg | €525.00 | 2-3 weeks | |
| (exo)-sTCO-COOH - 100 mg | €925.00 | 2-3 weeks |
Product information
-
Function
Trans-cyclooctene (TCO) – tetrazine click chemistry can be used for in vivo conjugation of two payloads. The click reaction can be employed for bioconjugations, immobilization, in vivo staining and/or in vivo fluorescent labeling. In addition, allylic functionalized TCO derivatives are known to release a payload after the conjugation reaction with various tetrazines.1
-
Mode of action
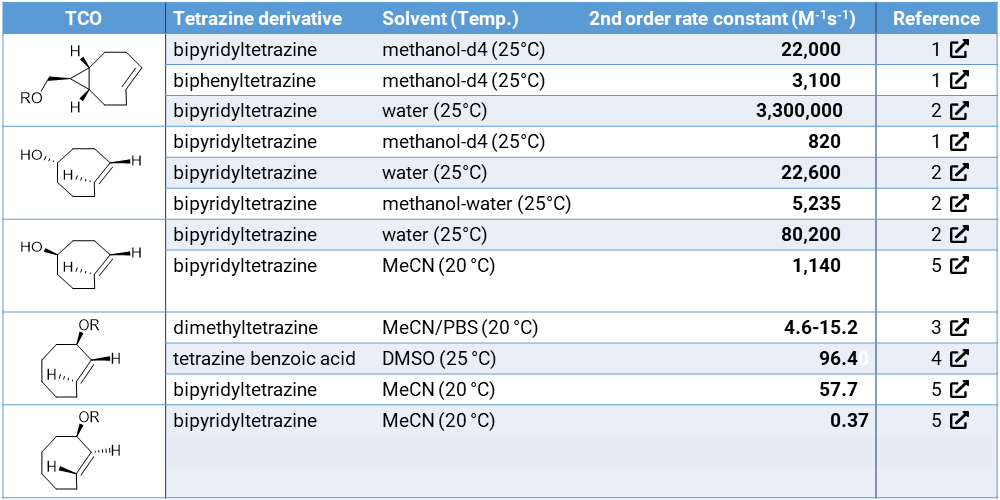
The reaction of tetrazines with trans-cyclooctenes (TCOs) covalently binds the two molecules without interfering with the native processes in living systems.2 The reactions of TCOs with tetrazines have exceptionally fast reaction rates of up to 3.300.000 M–1s–1 depending on the TCO structure.3 These properties make TCO click-chemistry a powerful tool for in vivo conjugations. Both the tetrazine and TCO can be charged with a payload (e.g. marker, antibodies, drugs) that can be joined in vivo. TCOs with a payload on the allylic position have been employed to release the payload after forming the click conjugate with tetrazine. The release kinetics are dependent on the stereochemistry of the TCO, the linking type of payload and the tetrazine derivative that is employed in the conjugation reaction.4
-
Applications
The application of trans-cyclooctene (TCO) based click chemistry varies from synthesis of nanomaterials to labeling and drug delivery strategies.56 The combination of TCO click chemistry with labeling dyes, radioactive isotopes, quantum dots or biomolecules such as proteins or nucleotides are already being explored.7891011 In preclinical studies of Positron Emission Tomography (PET) imaging with in vivo pretargeting, antibody-drug conjugates (ADCs) were successfully used for site-specific delivery of a radioactive cargo in mice.1213 Furthermore, the “click-to-release” capabilities of TCOs with allylic functionalization have successfully been employed to locally release Doxorubicin and the synthetic antineoplastic agent Monomethyl auristatin E (MMAE). The inactive drugs attached to TCOs was immobilized on tumor tissue and activated by tetrazine triggered release of the payload.11415
-
Handling
TCOs are sensitive to light. The product and reactions with the product should be shielded from light.
-
Biological Activity
TCOs have been used in cell cultures and mice for both imaging and therapy uses.141617
-
Chemical Information
CAS No.: n/a
SMILES: [H][C@@]12[C@@]([C@@H]2C(O)=O)([H])CC/C=C/CC1
Chemical formula: C10H14O2
Molecular weight: 166.22
Purity: > 95%
Identity: 1H NMR
Shipping temperature: 20°C
Storage temperature: -20°C
Recommended Products
| Name |
|---|
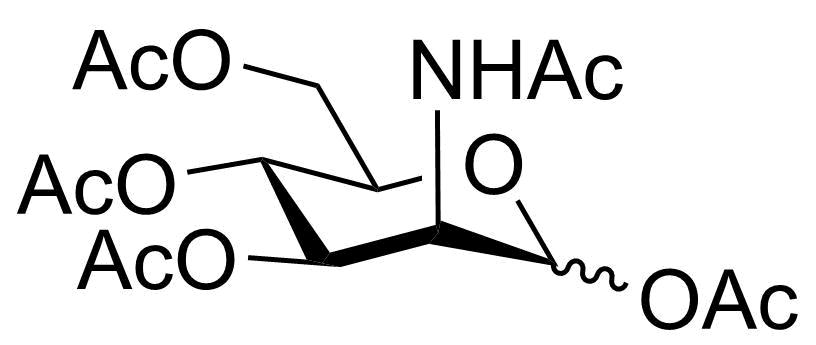
| P-GalNAc |
| (exo)-sTCO-CH2OH |
| 5-OAc-TCO |
| 3-OAc-TCO |
| P-ManNAc |
Calculator
Dissolve the required mass in your desired stock volume.
Dilute the required volume of your stock solution to the desired final volume.